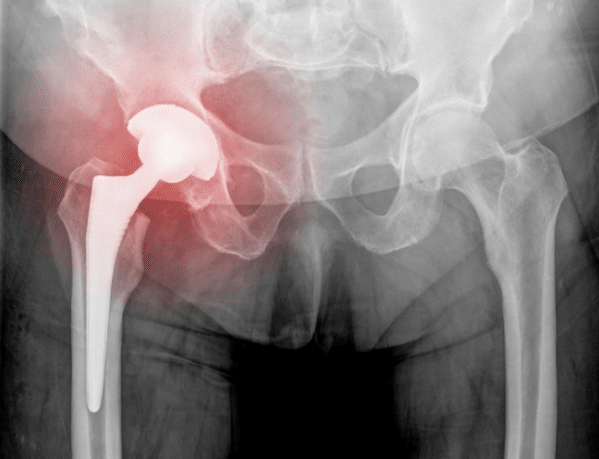
This is a two phased study consisting of a clinical follow up phase and a clinical outcomes phase.
Ceramic hip replacement studies.
This study is intended to gather medium 0 5 years and long term 6 10 years information regarding the performance and safety of the commercially available ceramax ceramic on ceramic total hip system.
They do not have any biological adverse effects or allergic responses for example.
The mean duration of the combined studies was 8 4 years while the mean age of the participants was 54 5 years.
Listing a study does not mean it has been evaluated by the u s.
Research conducted in 2015 reviewed five high qualitiy studies investigating the clinical outcome of people receiving an all ceramic hip implant.
In total 897 patients were included.
28 mm ceramic on ceramic acetabular cup total hip replacement study the safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
This is a two phased study consisting of a clinical follow up phase and a.
Ceramic hip replacement implants have functioned very well in laboratory studies and short term human investigations.
28mm ceramic on ceramic total hip replacement study study purpose this study is intended to gather medium 0 5 years and long term 6 10 years information regarding the performance and safety of the commercially available ceramax ceramic on ceramic total hip system.